PMA Zeolite – Effects, Safety, and Research
Everything about the effects of PMA Zeolite: toxin binding in the gut, strengthening of the intestinal barrier, and clinically proven detoxification with patented PMA technology.
What is PMA Zeolite?
PMA Zeolite from PANACEO is a patented, natural medical product based on high-quality clinoptilolite zeolite. Through the unique PMA activation process, the natural effects of the volcanic mineral are specifically enhanced for:
- optimal toxin binding in the gut, and
- a strengthened intestinal barrier and lasting support for gut health.
PMA Zeolite consists of clinoptilolite zeolite of volcanic origin, which is refined and activated using the proprietary PMA process (Patented Micro Activation). This process increases the internal surface area and optimizes the crystal structure, significantly enhancing its natural adsorption capacity.
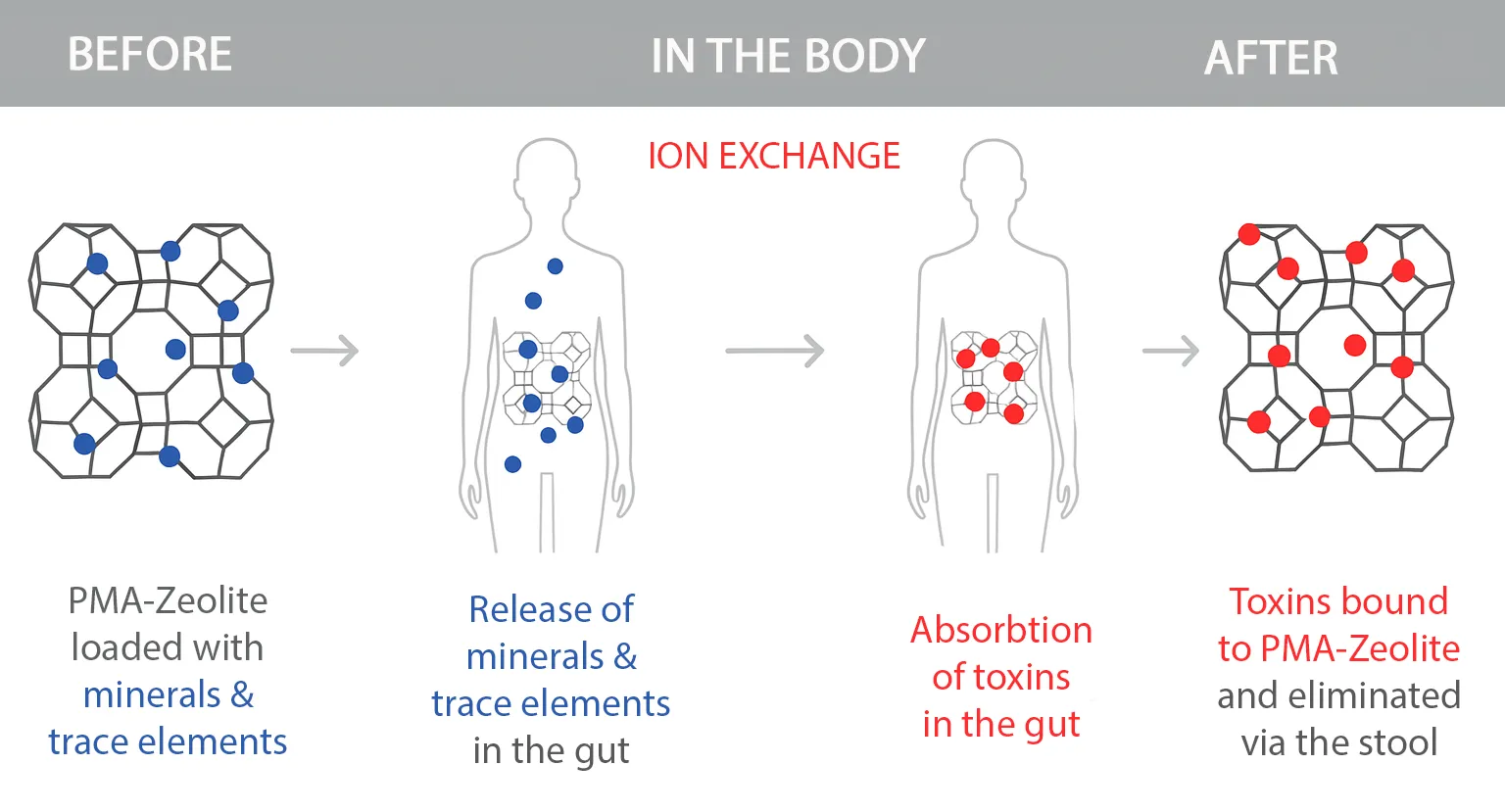
How Does PMA Zeolite Work in the Body?
PMA Zeolite acts like a microporous sponge in the digestive tract: it selectively binds toxins such as ammonium, lead, cadmium, and arsenic without removing essential nutrients from the body. At the same time, it releases important minerals like magnesium and calcium. The bound substances are naturally excreted through the stool.
- Binding of heavy metals and environmental toxins in the gut
- Support for the natural intestinal barrier
- Reduction of oxidative stress and inflammation
- Promotion of gut health and immune function
PMA Technology – The Unique Selling Point
Only PANACEO uses the patented PMA activation process, in which the zeolite lattice is mechanically activated. This significantly increases its adsorption capacity, as scientifically proven in independent clinical studies. No other provider uses this technology with comparable quality and documentation.
Who Is PMA Zeolite Suitable For?
- People with high environmental exposure or toxin load
- Individuals with irritable bowel syndrome, leaky gut, or chronic fatigue
- Athletes seeking to reduce lactate and oxidative stress
- As support during fasting programs or diets
- For general immune support and overall well-being
Safety & Quality Commitment
PMA Zeolite is certified as a Class IIb medical device according to ISO 13485 and bears the CE mark. With over 3 million products sold and more than 15 years of market experience, its long-term safety is well established.
The only known side effect is occasional constipation due to insufficient fluid intake. Therefore, it is recommended to drink enough water and to consult healthcare professionals before use in specific situations (e.g., during pregnancy or chemotherapy).
Clinical Studies & Scientific Evidence
The effectiveness and safety of PMA Zeolite have been demonstrated in numerous clinical studies and observational applications. These have shown positive effects on:
- Reduction of oxidative stress
- Strengthening of the intestinal wall barrier (anti-leaky gut)
- Relief for the liver and kidneys
- Support during physical or environmental stress
Zeolite was even used for radioactive detoxification after the Chernobyl nuclear disaster – an impressive example of its binding capacity.
Why PANACEO?
PANACEO is the market leader in PMA Zeolite and a pioneer in scientific research, collaborating with internationally recognized partners in medicine and science. From raw material selection to final quality control, the focus is on maximum purity, sustainability, and scientifically proven efficacy.